Technology

Diamond is a neural network of predictive analytics, machine learning, and artificial intelligence programming synergistically designed to identify the best possible cancer therapy targets.
Over 2 Billion Data Points

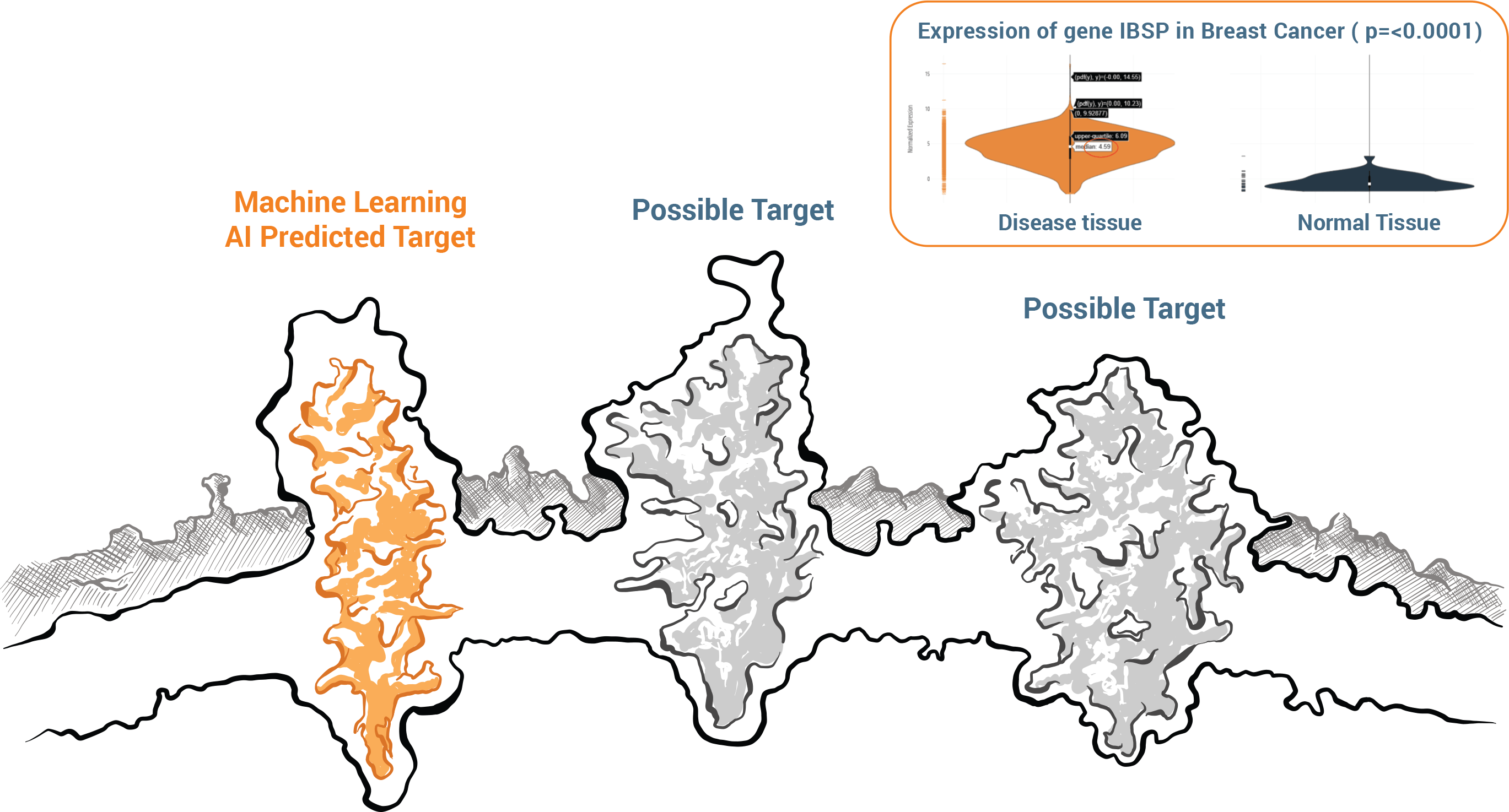
Identify Highly Expressed Genes
Diamond’s cognitive and deep learning capabilities extract information from our extensive digital library consisting of clinical studies, and genomic and proteomic datasets. Diamond harmonizes billions of data points and creates datasets allowing Kiromic to screen for new cancer targets, the limited number of which is one of the greatest roadblocks to effective CAR-T therapy development in the market today, especially for solid cancers.
Diamond will identify and prioritize lists of genes (biomarkers, wild type, mutant, isoform, etc.) that are highly and specifically expressed in the disease of interest including the analysis of disease distribution. It also maps out the exact portion of the gene that will elicit an immune response.
Perform Meta Analysis
Diamond performs meta-analysis and convolution studies while standardizing and normalizing data across multiple and variable experimental platforms, which allows for the visualization of consistent and accurate results in a user-friendly fashion.
Isoform Target Prediction
Cancer cells will down regulate or shed targets in order to avoid detection and destruction by T-cells (the immune system).
Alternative protein sequences, called “isoforms’, are generally much harder for tumors to hide from the immune system.
Target isoforms are protein variants of the same targets and include variations in the primary amino acid sequence that can change the final folded form of the target, increasing its ability to be recognized by T-cells.
To detect isoforms, Diamond shows a box plot of the expression of unique cancer targets such as target isoforms which are detected in the cancer genome tissue atlas, which are then correlated with a box plot of the matching isoforms in normal genotype-tissue expression data sets. Diamond then highlights the normal sequence of amino acids that are specific for the selected cancer isoforms.
Prediction Engine
Isoforms are alternative protein sequences, and Diamond AITM is able to distinguish between proteins (targets) on the surface of cancer cells and proteins on the surface of normal cells, which is important for decreasing the risk of toxicity.
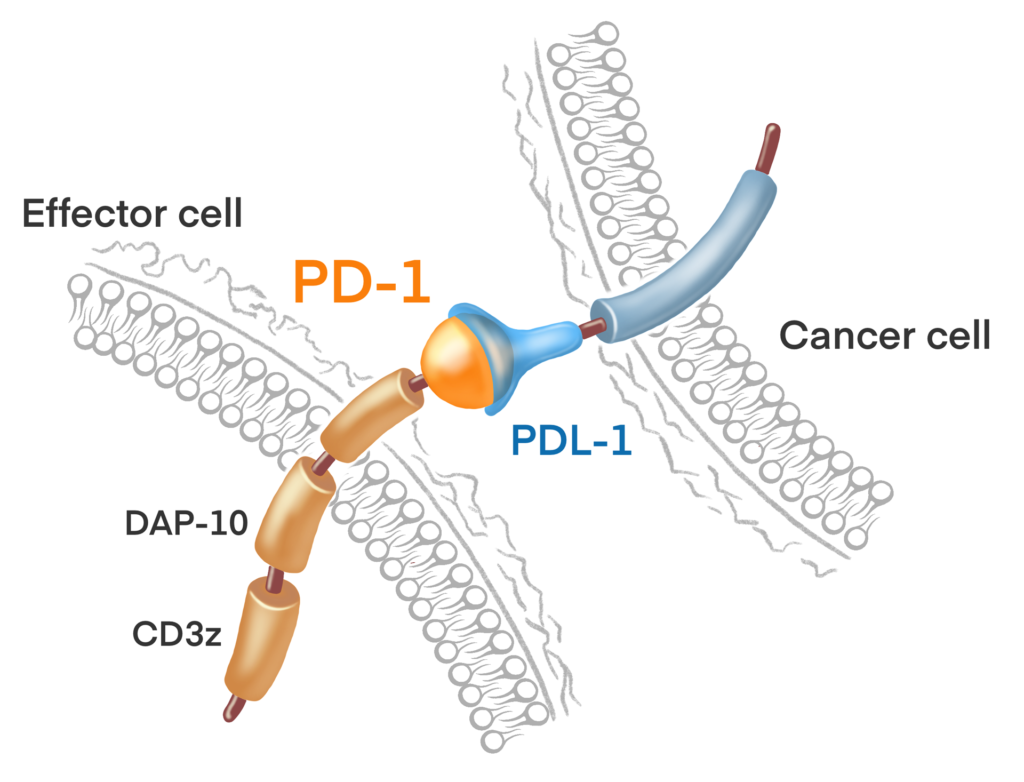
Immune Surveillance
The ALEXIS-PRO-1 clinical trial will study the impact of cellular checkpoint inhibitor therapy.
This is accomplished by genetically engineering gamma delta T-cells to bind with PD-L1 antigens on tumor cells, in much the same way that the FDA approved checkpoint inhibitors do.
The reason that checkpoint inhibitors are effective is they “bind and block” the ability of the tumor cell’s PD-L1 antigen (the tumor’s protective checkpoint) to “bind and turn-off” the attacking T-cells. Thus, they are called checkpoint inhibitors because they inhibit the ability of these tumor checkpoints to turn off attacking T-cells, resulting in improved immune activity against the tumor.
Kiromic’s approach is more novel. At Kiromic we designed our gamma delta T-cells to not only block the ability of PD-L1 like the checkpoint inhibitors do, but to also actually stimulate the gamma delta T-cells whenever this interaction/binding occurs between the protective tumor PD-L1 antigens and the gamma delta T-cells. This results in synergistically strong T-cell activity, as shown in Kiromic’s animal studies which demonstrate spectacular tumor cell killing capabilities. Accordingly, at Kiromic, we feel that this will be one of the key factors in the successful treatment of solid tumors with cellular therapy.